Our Services
1 Consultation Service
1.1 Engineering:
1.1.1 Facility Design and Review
1.1.2 Machine Design and Review
1.1.3 Utilities Design and Review
1.1.4 Computerized system Design and Review
1.1.5 EHS Environment, Health and Safety Assessment and Contingency
1.1.6 Energy Saving (Electrical – Water – Natural Gas – Operation consumables)
1.1.7 Consumption Monitoring and Reduction
1.1.8 Determine Level Of compliance with Good Engineering practice (GEP)
1.1.9 Criticality and Risk Assessments
1.1.10 Creating and Updating:
– Standard Operation Procedure (SOP)
– Standard Maintenance Procedure (SMP)
– Standard Cleaning Procedure (SCP)


1.2 QMS
1.2.1 Documentation Management System.
1.2.2 Change Control Management System.
1.2.3 Deviation and Defects Management System.
1.2.4 Risks Management System.
1.2.5 Communication Management System.
1.2.6 Calibration Management System.
1.2.7 Site Drawing Management System
1.2.8 Site Master File Preparation
1.3 Accreditation Preparation For:
1.3.1 The European Medicines Agency (EMA) GMP Inspection
1.3.2 The European Union (EU) GMP Inspection
1.3.3 The Food and Drug administration (FDA) Inspection
1.3.4 The World Health Organization (WHO) Inspection
1.3.5 The ISO 9001 – < Quality management systems > Inspection
1.3.6 The ISO 14001 – < Environmental Management > Inspection
1.3.7 The ISO 13485 – < Medical Device QMS requirements for regulatory purpose > Inspection
1.3.8 The ISO 45001 – < Occupational Health and Safety (OH&S) management system> Inspection
1.3.9 The ISO 17025 –
< GENERAL REQUIREMENTS FOR COMPETENCE OF TESTING AND CALIBRATION> Inspection
1.3.10 The ISO 22000
<Food Safety Management System – Requirements for any organization in the Food Chain> Inspection

1.4 Project
1.4.1 Project Management from Idea to operation
1.4.2 Project Coordination
1.4.3 Lean Six Sigma Project
2 CSV and Software Testing Services
2.1 CSV (Computer System Validation):
WE Offer complete Risk Based Validation for computerized Systems with all kind of development approaches,
For Systems Like
(Equipment – Lab. computerized Instruments – ETO – ERP – BMS – LIMS – Utilities – HPLC – IT).

2.2 Software Testing:
We offer Software testing service for Source code and Application Programs Interface (API) software.
2.2.1 White Box Testing
2.2.2 Black box Testing
2.2.3 Alfa testing
2.2.4 Beta Testing
3 Validation
We Offer Risk based Validation service with up to date testing activities to comply with your regulatory requirement and international organizations you are aiming to comply with.
We offer Creating/Review the Validation master plan for the site using criticality risk assessment tool for effective and risk based Plan which reflects on the accurate schedule and resources plan.
We can Create Plans and Protocols from scratch, or we can execute yours. You do not need to worry anymore about Risk assessments, URS, DQ, IQ, OQ, PQ, FAT, SAT and Protocol execution; our team will provide for you professional and fast services within the following points:
3.1 VMP Validation Master Plan
3.2 Changes control System
3.3 Equipment Validation
3.4 Facilities Validation
3.5 Utilities Validation
3.6 Electronic systems Validation
3.7 Transportation Validation
3.8 Packaging Validation
3.9 Analytical Method Validation
3.10 Spread Sheets Validation
3.11 Instrument Validation
3.12 Cleaning Validation
3.13 Process Validation
We Offer Validation for Products using one of the following approaches.
3.13.1 Traditional Process Validation Approach
3.13.2 Continuous Process Verification Approach
3.13.3 Hybrid Process Validation Approach
Our Validation Service is prepared and executed to comply with European and International Organizations Best Practices to maintain your facility valid.
4 Business Agility
Mega Project always had many changes during the Project phase, from changing/adding requirements to enhancing design to change the scope of the project due to budget. All of these usually reflect on either the project Quality or the Project Time line. This is a natural result when client requirements are not moving as fast as the project moves.
Our service here is to make sure that you will avoid such situation in your project by using the agile approaches one of the most advanced techniques globally in handling business projects.
5 Site Commissioning
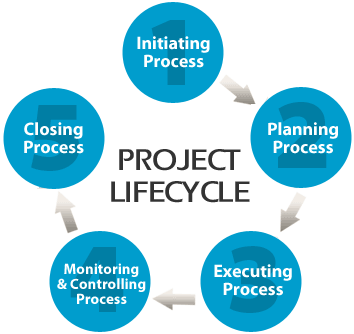
Site commissioning is one of projects that could result many conflicts during project phase to operation phase, that’s why we like to follow the PMI Project Management Institute to avoid that conflicts by implementing its nine Project management knowledge areas along the five processes groups for the project life time.
That secures for us a common ground to deal with different companies from different countries.
5.1 Initialization
5.2 Planning
5.3 Execution
5.4 Monitoring and Control
5.5 Closing
6 Site Remediation
Performing assessments and execute tests on your site to determine level of compliance with specific Regulatory entity or with one of our references, then Followed by Risk based mitigation plan with specific Actions with estimated time line.
Our support extends to coordinate and manage your project.
Our site Remediation service is performed by 5 different Phases
Phase 1: Data acquisition and analysis for current situation.
Phase 2: Set Plan for site to determine exact level of compliance.
Phase 3: Executing assessment and Testing activities (Re – Validation).
Phase 4: Set Mitigation Plan for Risks, Deviation, Defects and GAPs.
Phase 5: Execute the Mitigation Plan (Optional).

7 Decommissioning activities
Any unit has limited life time in the operation phase, and after that it is either replaced or removed from operation.
This process of removing or replacing a unit from Operation phase called Decommissioning.
We offer a Complete Safe Decommissioning process for your unit, through testing and documenting its status before it is removed from operation phase with compliance to regulatory and international requirements.
8 CE Mark Service
We facilitate and guide our clients to comply with CE Mark and Regulatory requirements for Medical Device, by providing the following services.
8.1 Preparation of CE Mark technical File
8.2 Preparation for CE Mark Inspection
8.3 Risk assessment
8.4 GAP analysis with ISO 13485:2016
8.5 Guidance through Medical device Directives, standards and references

9 Maintenance and Troubleshooting Service
We provide Troubleshooting, Maintenance, Cleaning service for your Facility, equipment’s and instruments according to latest techniques used globally.
Troubleshooting is art of detecting the root cause of your problem to fix it as fast as possible to avoid production breakdown loss, here come our mission.
Our Maintenance activities are performed with up-to-date technologies and GEP that make sure your unit will be performing as if it is brand new.
Cleaning is the basic Performance indicator for you facility and Equipment health, so we make sure that it looks clean to perform well and impress your employee and visitors.
We proudly provide these services for:
9.1 AHU and Ducts.
9.2 Air Dryer.
9.3 BMS
9.4 Water Pumps
9.5 Electric Motors
9.6 Chiller.
9.7 Centralized Air Conditioner
9.8 Split Air conditioner
9.9 Gardens
9.10 Facility Cleaning and Maintenance
10 Training Service

Our Training services is provided with Mentors have at least 10 years of experience in the field, or by young rising start trainer who had an innovative minds and extraordinary thinking methodology.
We aim to make sure that transferred information to the trainee is 100 % up to date and based on scientific facts, and our information references is always traceable to the origin.
We are providing Training in:












